Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Watanabe, Tadashi; Ito, Goichi*; Nakamura, J.*; Kono, Koji*; Ohashi, H.*
Proceedings of 3rd Korea-Japan Symposium on Nuclear Thermal Hydraulics and Safety (NTHAS-3), p.146 - 151, 2002/10
no abstracts in English
Suzuki, Satoru; Sato, Haruo; Ishidera, Takamitsu; Fujii, Naoki*; Kawamura, Katsuyuki*
JNC TN8400 2001-031, 44 Pages, 2002/05
In order to quantify effect of temperature on diffusivity of deuterated water (HDO) in compacted sodium-bentonite, through-diffusion experiments were conducted at elevated tempemture from 298 to 333 K. Kunipia F (Na-montmorillonite content  98 wt. %; Kunimine Industly Co.) was compacted to a dry density of 0.9 and l.35 Mg/m
98 wt. %; Kunimine Industly Co.) was compacted to a dry density of 0.9 and l.35 Mg/m . Since smectite flakes were perpendicularly oriented to a direction of compaction, anisotropy of diffusivity was investigated parallel and normal to the preferred orientation of smectite. Effective diffusion coeficient D
. Since smectite flakes were perpendicularly oriented to a direction of compaction, anisotropy of diffusivity was investigated parallel and normal to the preferred orientation of smectite. Effective diffusion coeficient D of HDO was larger for a diffusional direction parallel to the preferred orientation than normal to that for both dry densities. These results well agreed to the previously reported ones for tritiated water. Activation energies of D
of HDO was larger for a diffusional direction parallel to the preferred orientation than normal to that for both dry densities. These results well agreed to the previously reported ones for tritiated water. Activation energies of D in compacted bentonite increased with increasing dry density in the range of 19 - 25 kJ/mol which was slightly larger than that in bulk water (18 kJ/mol). This relationship can be considered to be due to both the pore structure development and high activation energy of water (18-23 kJ/mol) in the vicinity of smectite surface (within 2 nm) on the basis of molecular dynamics simulations.
in compacted bentonite increased with increasing dry density in the range of 19 - 25 kJ/mol which was slightly larger than that in bulk water (18 kJ/mol). This relationship can be considered to be due to both the pore structure development and high activation energy of water (18-23 kJ/mol) in the vicinity of smectite surface (within 2 nm) on the basis of molecular dynamics simulations.
Suzuki, Satoru; Sato, Haruo
JNC TN8410 2001-028, 36 Pages, 2002/03
For a safety assessment of the high-level radioactive waste disposal, effective diffusion coefficients (D ) of radionuclides in bentonite have been accumulated by the through-diffusion method. It has been found recently that experimental results on D
) of radionuclides in bentonite have been accumulated by the through-diffusion method. It has been found recently that experimental results on D s for several cations (cesium and strontium) by the fairly standard experimental method in JNC differ from those previously reported in several papers. Discrepancy can be considered to be due to different design of diffusion cell and system. In order to confirm influences of the experimental design on cation diffusivities in bentonite, a flow-through diffusion system was developed and several diffusion experiments were conducted.As a result, magnitude of D
s for several cations (cesium and strontium) by the fairly standard experimental method in JNC differ from those previously reported in several papers. Discrepancy can be considered to be due to different design of diffusion cell and system. In order to confirm influences of the experimental design on cation diffusivities in bentonite, a flow-through diffusion system was developed and several diffusion experiments were conducted.As a result, magnitude of D and its salinity dependence were relatively different between the standard and flow-through diffusion system. Since the latter system can control boundary conditions of the experiment more strictly than the standard method, we can conclude that the flow-through diffusion system provide correct results. In addition, we apply this flow-through diffusion system to a modification of controlling boundary condition during the experiment and to the diffusion experiment under controlled temperature.
and its salinity dependence were relatively different between the standard and flow-through diffusion system. Since the latter system can control boundary conditions of the experiment more strictly than the standard method, we can conclude that the flow-through diffusion system provide correct results. In addition, we apply this flow-through diffusion system to a modification of controlling boundary condition during the experiment and to the diffusion experiment under controlled temperature.
JNC TN8400 2000-012, 33 Pages, 2000/04
The redox condition of near-field is expected to affect the performance of engineered barrier system. Especially, the oxygen initially existing in the pore space of compacted bentonites strongly affects the redox condition of the near-field. For assessing the influence of the oxygen, the transport parameters of it in the compacted bentonite and consumption process should be known. Therefore, following researches were conducted. In order to understand the diffusion of dissolved oxygen (DO) in compacted bentonite and to predict the effect of DO, the effective diffusion coefficients of DO in compacted sodium bentonite were measured by electrochemistry. As the results, the following relationship between the dry density of compacted sodium bentonite and the effective diffusion coefficient of DO in compacted sodium bentonite was derived: De=1.53 0.13
0.13 10
10 exp(-2.15
exp(-2.15 0.24
0.24 10
10 p) where De is the effective diffusion coefficient (m
p) where De is the effective diffusion coefficient (m s
s ) of DO in compacted sodium bentonite and
) of DO in compacted sodium bentonite and  is the dry density (kg m
is the dry density (kg m ) of compacted sodium bentonite. The oxygen concentration in the bentonite is expected to be controlled by oxidation of pyrite as impurity in the bentonite. In order to investigate the above idea, the rates of pyrite oxidation by DO in compacted sodium bentonite were estimated from the experimental data on pyrite-bentonite systems usig the obtained effective diffusion coefficient of DO. The results show that the averages of the rate constants of pyrite oxidation by DO in the bentonite for dry densities of 0.8, 0.9, 1.0, 1.1 and 1.2
) of compacted sodium bentonite. The oxygen concentration in the bentonite is expected to be controlled by oxidation of pyrite as impurity in the bentonite. In order to investigate the above idea, the rates of pyrite oxidation by DO in compacted sodium bentonite were estimated from the experimental data on pyrite-bentonite systems usig the obtained effective diffusion coefficient of DO. The results show that the averages of the rate constants of pyrite oxidation by DO in the bentonite for dry densities of 0.8, 0.9, 1.0, 1.1 and 1.2 10
10 kgm
kgm were 1.38
were 1.38 0.32
0.32 10
10 , 1.10
, 1.10 0.24
0.24 10
10 , 1.16
, 1.16 0.35
0.35 10
10 , 9.36
, 9.36 2.23
2.23 10
10 and 7.48
and 7.48 1.92
1.92 10
10 ms
ms , respectively. The relationship between the dry density (
, respectively. The relationship between the dry density ( ) and the rate constant (k') was expressed as follows: k'=3.94
) and the rate constant (k') was expressed as follows: k'=3.94 1.06
1.06 10
10 exp(-1.33
exp(-1.33 0.28
0.28 10
10
 ) ...
) ...
Mihara, Morihiro; ; Ueta, Shinzo*; *
JNC TN8430 99-011, 27 Pages, 1999/11
In radioactive waste disposal, compacted Na-bentonite has been proposed for a buffer material. However, Na-bentonite would change to Ca-bentonite in the long term period. The change of Na-bentonite to Ca-bentonite might cause the change in the data concerning with nuclides migration properties such as permeability, sorption and diffusion. In this study, effective diffusion coefficients of HTO, Cs, I and C in compacted Ca-bentonite which was changed from Na-bentonite, Kunigel V1, were obtained and were compared to published those of Kunigel V1. In addition, effective diffusion coefficients for compacted Ca-bentonite with syncetic sea system water, SW, were obtained in order to investigate effect of solution composition. The magnitude of effective diffusion coefficients in Ca-bentonite are arranged in smaller order as Cs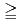 HTO
HTO I
I C. It is estimated that their effective diffusion coefficients are same those of Na-bentonite. About effect of solution composition, effective diffusion coefficients of HTO in 1.8g/cm
C. It is estimated that their effective diffusion coefficients are same those of Na-bentonite. About effect of solution composition, effective diffusion coefficients of HTO in 1.8g/cm dry density with SW were almost same values with distilled system water, DW. However, effective diffusion coefficients of HTO in lower density were smaller than values with DW. Regarding as effective diffusion coefficients of Cs in 1.8g/cm
dry density with SW were almost same values with distilled system water, DW. However, effective diffusion coefficients of HTO in lower density were smaller than values with DW. Regarding as effective diffusion coefficients of Cs in 1.8g/cm dry density, the effect of SW could not be observed as well as HTO. However, effective diffusion coefficients of I and C existing as an anion in pore water of bentonite increased by the reduction in the ion exclusion.
dry density, the effect of SW could not be observed as well as HTO. However, effective diffusion coefficients of I and C existing as an anion in pore water of bentonite increased by the reduction in the ion exclusion.
Sato, Haruo
JNC TN8400 99-065, 379 Pages, 1999/10
A database for diffusivity for a data setting of effective diffusion coefficients in rock matrices in the second progress report, was developed. In this database, 3 kinds of diffusion coefficients: effective diffusion coefficient (De), apparent diffusion coefficient (Da) and free water diffusion coefficient (Do) were treated. The database, based on literatures published between 1980 and 1998, was developed considering the following points. (1)Since Japanese geological environment is focused in the second progress report, data for diffusion are collected focused on Japanese major rocks. (2)Although 22 elements are considered to be important in performance assessment for geological disposal, all elements and aquatic tracers are treated in this database development considering general purpose. (3)Since limestone, which belongs to sedimentary rock, can become one of the natural resources and is inappropriate as a host rock, it is omitted in this database development. Rock was categorized into 4 kinds of rocks; acid crystalline rock, alkaline crystalline rock, scdimentaly rock (argillaceous/tuffaceous rock) and sedimentary rock (psammitic rock/sandy stone) from the viewpoint of geology and mass transport. In addition, rocks around neutrality among crystalline rock were categorized into the alkaline crystalline rock in this database. The database is composed of sub-databases for 4 kinds of rocks. Furthermore, the sub-databases for 4 kinds of the rocks are composed of databases to individual elements, in which totally, 24 items such as species, rock name, diffusion coefficients (De, Da, Do), obtained conditions (method, porewater, pH, Eh, temperature, atmosphere, etc.), etc. are input. As a result of literature survey, for De values for acid crystalline rock, totally, 207 data for 18 elements and one tracer (hydrocarbon) have been reported and all data were for granitic rocks such as granite, granodiorite and biotitic granite. For alkaline crystallinc rock, ...
Sato, Haruo
JNC TN8400 99-062, 16 Pages, 1999/10
Effective diffusion coefficients (De) for Ni , Sm
, Sm , Am
, Am and SeO
and SeO were measured as a function of the ionic charge of diffusion species to quantitatively evaluate the effect of ionic charge in compacted bentonite. The De measurements for Ni
were measured as a function of the ionic charge of diffusion species to quantitatively evaluate the effect of ionic charge in compacted bentonite. The De measurements for Ni and Sm
and Sm were carried out for a bentonite dry density of 1.8 Mg
were carried out for a bentonite dry density of 1.8 Mg m
m with a simulated porewater condition of pH5
with a simulated porewater condition of pH5 6 by through-diffusion method. The De values for SeO
6 by through-diffusion method. The De values for SeO were measured for a bentonite dry density of 1.8 Mg
were measured for a bentonite dry density of 1.8 Mg m
m with a simulated porewater condition of pH11. The De measurements for Am
with a simulated porewater condition of pH11. The De measurements for Am were carried out for the dry densities of 0.8, 1.4 and l.8 Mg
were carried out for the dry densities of 0.8, 1.4 and l.8 Mg m
m with a porewater condition of pH2 in order to check cation exclusion. Sodium bentonite, Kunigel-V1 was used for those measurements. For the measurements of Am, H-typed Kunigel-V1 which interlayer ion (Na
with a porewater condition of pH2 in order to check cation exclusion. Sodium bentonite, Kunigel-V1 was used for those measurements. For the measurements of Am, H-typed Kunigel-V1 which interlayer ion (Na ) was exchanged with H
) was exchanged with H was used, because the experiments are carried out for a low pH range. The order of obtained De values was Sm
was used, because the experiments are carried out for a low pH range. The order of obtained De values was Sm
 Ni
Ni
 Am
Am
 SeO
SeO . These De values were compared to those reported to date. Consequently, the order of De values was Cs
. These De values were compared to those reported to date. Consequently, the order of De values was Cs
 Sm
Sm
 HTO
HTO  Ni
Ni
 anions (I
anions (I , Cl
, Cl , CO
, CO , SeO
, SeO TcO
TcO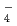 , NpO
, NpO CO
CO , UO
, UO (CO
(CO )
)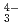 ), showing a tendency of cations
), showing a tendency of cations  HTO
HTO  anions. Only the De values of Am
anions. Only the De values of Am were approximately the same degree as those of anions. The reason that the De of Ni
were approximately the same degree as those of anions. The reason that the De of Ni was lower than that of HTO may be because the free water diffusion coefficient (Do) of Ni
was lower than that of HTO may be because the free water diffusion coefficient (Do) of Ni is about 1/3 of that of HTO. The cause that the De of Am
is about 1/3 of that of HTO. The cause that the De of Am was approximately the same degee as those of anions may be because the Do of Am
was approximately the same degee as those of anions may be because the Do of Am is about 1/3 of that of HTO and that Am
is about 1/3 of that of HTO and that Am was electrostatically repulsed from the surface of bentonite by cation exclusion. The formation factors (FF), calculated normalizing Do, were in the ...
was electrostatically repulsed from the surface of bentonite by cation exclusion. The formation factors (FF), calculated normalizing Do, were in the ...
Yamaguchi, Tetsuji;
Journal of Contaminant Hydrology, 35, p.55 - 65, 1998/00
Times Cited Count:29 Percentile:62.91(Environmental Sciences)no abstracts in English
Fukuda, Kenji; Ozaki, Yusuke; Murakami, Hiroaki; Itai, Kaori*; Ishibashi, Masayuki; Sasao, Eiji
no journal, ,
Matrix diffusion is one of the important for evaluating solute transport in crystalline rock. In this study, effective diffusion coefficient, porosity, and resistivity of crystalline rock were measured. There was a positive correlation between the formation factor calculated from the resistivity measurement and the effective diffusion coefficient calculated from the transmission diffusion test. Therefore, the effective diffusion coefficient can be estimated from the resistivity measurement of crystalline rock.